NRC. 2015. A Framework for Assessing Effects of the Food System. National Research Council, National Academies Press. 19 pages.
Nitrogen (N) is essential for agricultural productivity, but in its more reactive forms, it can pose significant threats to humans and the environment. Quantifying the abundance of nitrogen in different chemical forms and understanding its pathways through soil, air, water, plants, and animals under different management scenarios are essential to minimize threats to human health and environmental quality. Nonetheless, studying multiple forms of nitrogen in the environment presents many challenges and calls for the use of a systems analysis framework.
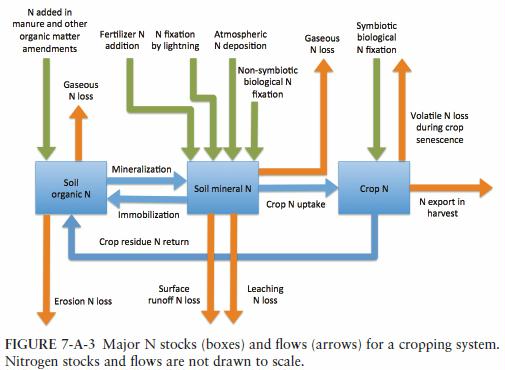
Nitrogen (N) is the most limiting element for plant growth in many ecosystems, despite being the most plentiful element in the earth’s atmosphere. In its most abundant form, gaseous dinitrogen (N2), N is unavailable to most organisms. However, following transformation to other forms, especially nitrate (NO3–) and ammonium (NH4+), N becomes highly reactive in the biosphere and can be highly mobile in water and air. Nitrogen is a key component of proteins in both plants and animals, including the enzymes responsible for photosynthesis and other critical biological reactions, and the muscles used for movement and other body functions. Consequently, most crops, especially cereals, require sizable supplies of N to yield well, and livestock and poultry need a diet rich in N to produce large quantities of milk, eggs, and meat. Agriculture now uses more reactive N than does any other economic sector in the United States and is also the sector responsible for the greatest losses of reactive N to the environment, where N has multiple unintended consequences, including threats to human health, degradation of air and water quality, and stress on terrestrial and aquatic organisms. Because reactive N strongly affects crop production and farm profitability, as well as human health and environmental quality, managing N efficiently and in an environmentally harmonious manner is a critically important component of agricultural sustainability.
Mineral N fertilizers produced through the Haber-Bosch process constitute the single greatest source of reactive N introduced into the United States, with about 11 teragrams (Tg) of fertilizer N being used in U.S. agriculture each year. (A teragram is the equivalent of 1 billion kilograms (= 2,204,622,622 pounds = 1,102,311 short tons)
Mineral forms of N fertilizer are energetically expensive to synthesize (57 MJ fossil energy/kg N) and sensitive to increases in the price of natural gas used in their production.
Thus, the fact that typically only 40% to 60% of applied N fertilizer is absorbed by crop plants implies large agronomic, economic, and energetic inefficiencies, as well as a large potential for excess N to move downstream and downwind from crop fields.
The exact fate of N fertilizer is heavily dependent on farm management decisions influencing N cycle processes, including crop selection, irrigation management, and the rate, formulation, placement, and timing of fertilizer applications. The fate of fertilizer N also can be highly dependent on weather conditions, especially precipitation patterns.
In addition to the application of mineral fertilizers, N may enter crop fields by several other pathways. Biological fixation of atmospheric N2 by microbes associated with the roots of leguminous crops like soybean and alfalfa (symbiotic fixation) adds about 8 Tg N per year to U.S. agroecosystems (EPA, 2011). Additional pathways by which reactive N is introduced into agroecosystems include lightning, fixation by nonsymbiotic microbes living in soil, and atmospheric deposition. The former two processes are responsible for adding only small quantities of N; the latter input can be locally important.
About 6.8 Tg of N is present in manure produced each year in the United States, but of that quantity, only 0.5 to 1.3 Tg N is applied to cropland and 3.7 Tg N is deposited on pastures and rangelands , indicating that a substantial proportion of manure N is not recycled effectively.
Moreover, manure application rates vary greatly among fields, with most fields receiving none and some receiving high rates.
Consequently, excessive concentrations of nutrients, especially phosphorus and N, can occur in the vicinity of concentrated animal feeding operations and can lead to water pollution.
Nitrogen can be lost from soil via
- Leaching, runoff, and denitrification are critical components of agroecosystem N dynamics, farm profitability, and environmental quality.
- As gaseous ammonia emitted from fertilizer and manure applied to the soil
- Senescing crops.
- Erosion of topsoil and the organic forms of N it contains constitutes another pathway for N loss from agroecosystems.
- Harvesting large amounts of crop residue. This can deplete soil organic matter and the lack of protective soil cover may result in increased amounts of N lost through erosion and runoff .
- Magnitudes of various N losses from agroecosystems are highly variable in space and time, and they are strongly influenced by weather conditions and management practices.
Human Health and Environmental Concerns
Reactive N released from agroecosystems is responsible for a number of adverse public health and environmental effects. Four of the most salient effects for the United States are noted here.
Drinking water contamination
Nitrate coming from farmland is an important contaminant of drinking water in many agricultural regions (EPA, 2011). It constitutes a potential health threat due to its ability to (1) induce methemoglobinemia, a condition in which the oxygen-carrying capacity of blood is inhibited; (2) promote endogenous formation of N-nitroso compounds, which are carcinogens and teratogens; and (3) inhibit iodine uptake, thereby inducing hypertrophic changes in the thyroid.
These health concerns are not restricted to members of the farm population. Nitrate contamination of surface water is common in the Corn Belt and is a recurrent challenge to cities such as Des Moines, Iowa, which draws drinking water from the Raccoon and Des Moines Rivers, both of which drain intensively farmed areas. After repeatedly violating the U.S. Environmental Protection Agency’s (EPA’s) drinking water standard of 10 mg L–1 for nitrate-nitrogen, and challenged by increasing levels of nitrate in its source water, the Des Moines Water Works constructed the largest ion exchange nitrate removal facility in the world in 1991. The need for this facility, which provides service to 500,000 people, has not abated, as record high levels of nitrate were encountered in Des Moines’ drinking water sources in 2013.
Nitrate also poses a significant threat to groundwater used for drinking water. A recent report focusing on the Tulare Lake Basin and Salinas Valley of California, which together contain 40% of the state’s irrigated cropland and more than 50% of its dairy cattle, found that nitrate poses a significant threat to the health of rural communities dependent on well water, with nearly 1 in 10 people in the two regions now at risk. The report identified agricultural fertilizers and animal wastes as the largest sources of nitrate in groundwater in the areas investigated
Eutrophication and hypoxia
Reactive N in water draining from agricultural regions can be responsible for eutrophication of freshwater bodies and hypoxia in coastal waters. High levels of N in water stimulate harmful algal blooms, leading to suppression of desired aquatic vegetation, and when the algae die, their subsequent decomposition by bacteria leads to large reductions in dissolved oxygen concentrations, with concomitant reductions in populations of shellfish, game fish, and commercial fish. Eutrophication and hypoxia effects are often spatially separated from their causes. For example, an estimated 71% of the N entering the northern Gulf of Mexico, the largest hypoxic zone in the United States and the second largest hypoxic zone worldwide, comes from croplands, rangelands, and pastures upstream in the Mississippi River Basin, with 17% of the total N load coming from Illinois, 11% from Iowa, and 10% from Indiana. Thus, because of the mobility of reactive N, agricultural practices and land uses in one region can affect water quality, recreational activities, and economic sectors like fisheries hundreds of miles downstream.
Greenhouse gas loading
Agricultural practices, principally fertilizer use, are responsible for about 74% of U.S. emissions of nitrous oxide (N2O), a greenhouse gas with a global warming potential 300-fold greater than that of carbon dioxide. Although the agricultural sector is responsible for only 6.3% of total U.S. greenhouse gas emissions, it is notable that agricultural emissions can offset efforts to use agricultural systems to mitigate climate change by sequestering carbon dioxide or providing alternative energy sources. Nitrous oxide emissions from agriculture also are notable as illustrations of how practices taking place locally on farmlands can have global scale effects.
Ecological and human health effects of ammonia and other NHx-N emissions
In 2002, the United States emitted 3.1 Tg of N into the atmosphere as ammonia and other NHx-N compounds, with agricultural practices, principally manure and fertilizer management, estimated to be responsible for 84% of that total. Most of these emissions are deposited within 1,000 km downwind as ammonia or ammonium in rainwater and aerosols. Ammonia emissions can lead to the formation of fine inorganic particulate matter (PM2.5) as ammonium-sulfate-nitrate salts, which are a factor for premature human mortality.
Deposition of reactive N from the atmosphere can acidify soils and waters and alter plant and soil community composition in grasslands and forests, leading to reductions in overall biological diversity and increases in the abundance of certain weedy species. Like the movement of reactive N in water from agricultural regions to coastal ecosystems, the aerial movement and deposition of NHx-N compounds illustrates that agriculture’s impact on the environment can extend into other ecosystems that may be located considerable distances from farmlands.
Using models of ammonia sources and transport and PM2.5 formation and deposition, Paulot and Jacob (2014) calculated the quantities of atmospheric ammonia and PM2.5 that are related to U.S. food exports and the associated impacts of these pollutants on human health. They concluded that over the study period of 2000 to 2009, 5,100 people died annually due to these emissions, incurring a cost of $36 billion. This value greatly exceeded the net value of the exported food ($23.5 billion per year). The investigators noted that these human health and economic costs indicated “extensive negative externalities,” and that taking into account other environmental impacts of agriculture, such as eutrophication, loss of biodiversity, and greenhouse gas emissions, would further diminish the value of agricultural production and exports.
Policy and Educational Considerations
Environmental quality and human health concerns related to the use of N for crop production have important policy dimensions.
In an analysis of 29 watersheds covering 28% of the United States, Broussard et al. (2012) noted that increases in federal farm program payments were significantly correlated with greater dominance of cropland by corn and soybean, more expansive fertilizer applications, and higher riverine nitrate concentrations.
They suggested that federal farm policies, expressed through farm payments, are a potent policy instrument that affects land-use decisions, cropping patterns, and water quality. Based on focus group interviews with farmers and residents of the Wells Creek and Chippewa River watersheds in Minnesota, Boody et al. (2005) noted that recent federal programs have encouraged the production of a narrow set of commodity crops while discouraging diversified agriculture and conservation efforts that better protect environmental quality. Similarly, Nassauer (2010, p. 190) observed that “for more than 50 years, production subsidies have vastly exceeded conservation spending––by almost ten times today—and this ratio has been clearly understood by farmers making production decisions.
Consequently, fewer opportunities exist for reducing N emissions to air and water from arable croplands through the increased use of conservation buffer strips and grasslands, reconstructed wetlands, and diversified cropping systems that include hay and other non-commodity crops.
Federal energy policies that have promoted ethanol production from corn grain have been linked to reactive N emissions. Donner and Kucharik (2008) used process-based models to simulate hydrological and nutrient fluxes in the Mississippi River Basin under different corn production scenarios. They found that the increase in corn cultivation required to meet the federal goal of producing 15 to 36 billion gallons of renewable fuels by the year 2022 would increase average annual discharge of dissolved inorganic N into the Gulf of Mexico by 10 to 34 percent.