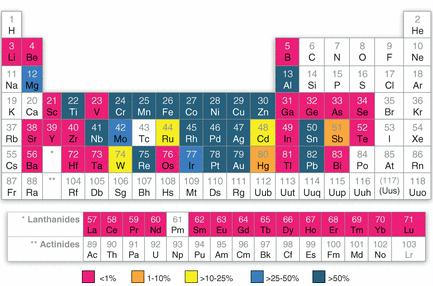
 Global estimates of end-of-life recycling rates for 60 metals and metalloids. Source: Reck, B. K. et al. 2012. Challenges in Metal Recycling. Science 337: 690-695
Global estimates of end-of-life recycling rates for 60 metals and metalloids. Source: Reck, B. K. et al. 2012. Challenges in Metal Recycling. Science 337: 690-695Preface. This is a post about why rare and critical metals aren’t recycled at all or at best, just a small percent. Basically it is still cheaper to mine them from scratch than to try to separate them out from electronic devices, and often impossible since they are an alloy or embedded with other metals that chemicals, heat, pressure and other techniques can’t separate out.
Mining and smelting ores is incredibly energy intensive. As ore quality declines, it will require more and more energy to crush the rock to get the metals out. But oil quality is declining too (tar sands, fracked oil, and Venezuelan heavy oil require so much energy to process the energy return is very low. And worse yet, oil is declining and will get more scarce and expensive (world peak oil production peaked in 2018). Electric mining trucks? Ha. Most sites are too far from the grid, and what electricity they do have comes from diesel powered generators.
Recycling harms health. Especially lead. One in three of the world’s 2.4 billion children under age 20 has a blood level exceeding what would trigger public health alarms in the U.S. The potent neurotoxin can reduce a child’s intelligence test score and cause other health problems; lead poisoning is blamed for nearly $1 trillion of lost lifetime earnings. Most lead enters the environment through poorly regulated smelters that recycle car batteries. Lead poisoning has worsened considerably during the past 2 decades because car sales in those countries have tripled, the report says. Scientists consider no amount of lead exposure safe, but the U.S. Centers for Disease Control and Prevention has set the threshold for action at 5 micrograms per deciliter—the level met or exceeded in 800 million children worldwide (Science 2020).
Alice Friedemann www.energyskeptic.com Author of Life After Fossil Fuels: A Reality Check on Alternative Energy; When Trucks Stop Running: Energy and the Future of Transportation”, Barriers to Making Algal Biofuels, & “Crunch! Whole Grain Artisan Chips and Crackers”. Women in ecology Podcasts: WGBH, Financial Sense, Jore, Planet: Critical, Crazy Town, Collapse Chronicles, Derrick Jensen, Practical Prepping, Kunstler 253 &278, Peak Prosperity, Index of best energyskeptic posts
* * *
The more intricate a product and the more minerals used, the better it will perform, but the more difficult it is to recycle the metals essential to making it work so well to begin with
With infinite amounts of energy, money, and time metals could be recycled. But in the real world it doesn’t happen due to the high cost, complex processes, and large amount of energy it takes to separate material, as well as poor recycling technologies, product design, and social behavior.
Less than 1% of 34 rare and critical metals are recycled. These metals are essential for microchips, solar PV, consumer electronics — pretty much all high-tech products have them.
But it is simply thermodynamically impossible to separate and recover many of them since they’re used in such tiny amounts for extremely precise purposes, and mixed with other rare metals (Bloodworth 2014, Reck 2012).
Metals such as tantalum, gallium, germanium, and rare-earth elements are oxidized and lost in the smelter slag (Hageluken 2012).
The most commonly recycled metals are also the cheapest and most abundant on the planet, such as steel, aluminum, copper, zinc, lead, and nickel, with rates often over 50%. This high recovery rate is due to their presence in relatively pure form in large amounts in products. But even these are reused 2 or 3 times before being lost to landfills.
The methods to recover rare metals are far more complex. These metals are used in myriad applications, from cell phones to satellites. Up to 60 different elements go into the manufacture of microprocessors and circuit boards (Gunn 2013), usually in tiny quantities and often in combinations not found in nature.
The need to recycle is obvious — only by doing so can the life of these resources be extended to future generations, since ores continue to be of lower and lower grades that need more energy to extract while at the same time the oil, coal, and natural gas energy needed to extract minerals is diminishing.
Even the valuable precious metals only have a recycling rate of 60%, and just a 50% recovery of platinum, palladium, and rhodium from auto catalytic converters because so many old cars are exported to developing countries that don’t have recovery technology. For the same reason, when it comes to the platinum group metals in electronics, the rate is even lower, just 5 to 10%.
Many of these metals are highly toxic to plants and animals, yet they’re recycled at very low rates, one of the reasons a fifth of China’s arable land is polluted with toxic heavy metals (Chin 2014). One of the worst, cadmium, is mainly recycled from nickel-cadmium batteries, but at very low rates. Mercury recovery is at best 10-20% recovered from fluorescent light bulbs. Ecotoxicity from metal-containing nanomaterials is also a problem.
The US Geological survey estimates the average recycling rate for most metals is 50% (Papp 2007). This means that after just 4 recycles, we’ve lost 95% of the original amount.
This is a shame, because most metals used in electronic devices use rare earth metals for which there is no substitution with the same efficiency. And a shortage of some looms, the reserves-to-production ratio for gallium, germanium, and indium (indispensable for touch screens and other displays) is estimated to be less than 20 years of supply (Frondel et al. 2006). Less than 1% of rare elements are cycled from e-waste. It’s too expensive to recycle them, so they end up in furnaces burned up with the plastic boards containing them. The few places rare earth metals are recovered don’t want to share their proprietary methods with other potential recyclers.
Worse yet, planned obsolescence is alive and well. Objects are still designed to break down and impossible to repair, forcing customers to buy a new one.
Thermodynamics is the ultimate limitation at the final processing stage and can’t be separated out.
Material is lost along the way
- Initial collection: a fraction of overall electronic equipment is turned into recycling centers, the percent depends on social and government factors.
- Recycling centers: much of the electronic waste is sent to countries that have inadequate recycling facilities
- Preprocessing & Sorting – some components are too much effort to take apart, so they’re discarded. Nor is there enough material to justify the cost of machine recycling technology.
- Recycling technology: Usually just shredding, crushing, magnetic sorting is done. It’s too expensive to recover even more with lasers, near-infrared, or x-ray sorting.
- Product design: often makes it hard to separate products out, such as laminated permanent magnets in computers.
- Smelter – the easier, larger, most common metals make it to the smelter, i.e. iron, aluminum, etc. Not all material that was collected and could be smelted reaches the smelters, especially if smelters are distant.
Downcycling (Bardi 2014).
One of the big problems with waste recycling is known as “downcycling”, because the recycled material isn’t as good as the original product. Consider steel. Although we recycle 68% of iron and steel, the problem is that the original steel was custom-made for a particular application to be hard or strong or corrosion resistant. This is done by adding other elements and creating an alloy with the needed properties (i.e. chromium, cobalt, silicon, manganese, vanadium, and other elements). Trying to control the concentration of these other metals during recycling is so complex and expensive that it usually isn’t done. As a result, recycled steel is lower-quality since it can’t be counted on to be as hard, strong, or corrosion-resistant and can’t be re-used in many industries.
Every time paper is recycled its fibers get shorter which makes an inferior product. Downcycling prevents perpetual recycling.
Similarly, when different kinds of plastics are mixed the resulting plastic has poor mechanical properties with limited uses.
Beverage cans have magnesium mixed in with the aluminum, requiring several more stages of separation to be transformed back into pure aluminum.
In all cases, recycling grows more difficult as the recycled fraction increases or higher performance is needed from the recycled material.
In the end, that takes more money and energy, which is why economically justifiable recycling is far less than 100%. Rare metals like indium and gallium are not recycled at all.
Biellow, David. 9 Aug 2012. Recycling Reality: Humans Set to Trash Most Elements on the Periodic Table. Scientific American
Almost all lead is recycled, among the only elements on the periodic table to earn that distinction. With good reason, mind you: the soft metal is a potent neurotoxin known to impact children’s brain development, among other nasty health effects. Today, nearly all lead is used in batteries (though it was once put into gasoline, leading to widespread contamination, and, in places like Afghanistan, still is.) Most of this dangerous element is now endlessly cycled from battery to battery, thanks to stringent regulations (though enough of it ends up being improperly recycled to constitute one of the world’s worst pollution problems.)
In principle, all metals are infinitely recyclable and could exist in a closed loop system, note the authors of a survey of the metals recycling field published in Science on August 10. There’s a benefit too, because recycling is typically more energy-efficient than mining and refining raw ore for virgin materials. Estimates vary but mining and refining can require as much as 20 times the amount of energy as recycling a given material. Think about it: a vast amount of energy, technology, human labor and time are expended to get various elements out of the ground and then that element is often discarded after a single use.
Lead is not alone in being recycled, of course. Aluminum, copper, nickel, steel and zinc all boast recycling rates above 50% (though not much above 50%). The same principles can be usefully applied to other materials, like plastics. After all, these ubiquitous polymers are made from another scarce resource oil and many are, in principle, recycleable. Yet, the overall recycling rate for plastics, grouped as a whole, is only 8% (as of 2010, per EPA numbers.) Take the case of polypropylene (or #5 plastic if you’re checking the bottom of your food containers). The bulk of this polymer that gets recycled comes from car batteries. It is, in essence, tagging along with the lead. In other cases water bottles, yogurt cups, you name it it simply disappears into the nation’s landfills.
Meanwhile, the majority of elements on the periodic table and we use almost every element on the periodic table for something or other are also nearly completely unrecycled.
As an example, industrial ecologists Barbara Reck and T.E. Graedel of Yale University compare the fates of nickel versus neodymium. Nickel is ubiquitous, particularly as an alloy for steel. Of the 650,000 metric tons of the silvery-white metal that reached the end of its useful life in one product in 2005, roughly two-thirds were recycled. And that recycled nickel then supplied about one-third of the demand for new nickel-containing products. That means the overall efficiency of human use of nickel approaches 52%. Not bad, but there’s room for improvement, given that almost half of all nickel is only used once before it is discarded.
Nearly 16,000 metric tons of neodymium a so-called rare earth metal were employed in 2007, mostly for permanent magnets in everything from hybrid cars to wind turbines. Roughly 1,000 metric tons of the element reached the end of its useful life in one product or another and “little to none of that material is currently being recycled,” the survey authors note. This despite the fact that a “rare earth crisis” stems from China’s near monopoly of the neodymium trade.
Mining for neodymium is not benign (which is why the world lets China monopolize its production). And it’s not just neodymium. Mining waste or tailings, leach ponds, slurries and the like are among the world’s largest chronic waste problems. North America alone produces 10 times as much mining waste as it does the municipal solid waste (as it’s known) from all the neighborhoods in the U.S. Much of that is just rock, sand and dust the mountaintop in mountaintop removal mining. And mined products also cause waste further down the product line, such as the ash leftover after the coal is burned (the U.S.’s largest single form of waste).
This issue of profligate use gets worse: we are currently making this problem even harder to solve. How? One word: gadgets. In most gadgets you can think of, tiny amounts of rare elements are used to enhance functionality. As the industrial ecologists write in Science: “The more intricate the product and the more diverse the materials set it uses, the better it is likely to perform, but the more difficult it is to recycle so as to preserve the resources that were essential to making it work in the first place.” It’s as true of iPhones as it is of photovoltaic panels and none of them have shown much success in being recycled. “End of life losses will also increase sharply soon,” unless something changes, the industrial ecologists warn.
Then there are the alloys, where thermodynamics dictate that the alloying element is almost always going to be lost due to the difficulty of separation. That means the chromium used in stainless steel will usually lose its luster, for example. Worse, this form of contamination can mean that the recycled alloy can’t be re-used manganese-aluminum alloys are unsuitable once recycled for 95 percent of the uses for aluminum. As a result, “current designs are actually less recycleable than was the case a few decades ago,” the authors note. Perhaps the use of such metal combinations should be minimized?
In the end, our approach to recycling is bizarre, given our resources. “Few approaches could be more unsustainable,” Reck and Graedel write. In the end, we’ll learn to reuse all the elements of the periodic table, or we’ll lose elements to use.
REFERENCES
Bardi, Ugo. 2014. Extracted: How the Quest for Mineral Wealth Is Plundering the Planet. Chelsea Green Publishing.
Bloodworth, A. 2014. Track flows to manage technology-metal supply. Recycling cannot meet the demand for rare metals used in digital and green technologies. Nature 505: 19-20.
Chin J, et al. 2014. China details vast extent of soil pollution. About a fifth of nation’s arable land is contaminated with heavy metals. Wall Street Journal.
Frondel, M., et al. 2006. Trends der angebots- und nachfragesituation bei mineralischen rohstoffen. Federal ministry of economics and energy.
Gunn, A. G. 2013. In Proc. 12th Bienn. Soc. Geol. Appl. Miner. Depos. Meet (SGA, 2013)
Hageluken, C et al. 2012. Precious Materials Handbook, Ch 1. Hanua-Wolfgang.
Papp, J. F. 2007. 2005 minerals yearbook: recycling—metals. U.S. Geological Survey.
Pihl, E., et al. 2012. Material constraints for concentrating solar thermal power. Energy 44: 944-954
Reck, B. K. et al. 2012. Challenges in Metal Recycling. Science 337: 690-695
Science. 2020. News at a glance. One in three children poisoned by lead.
Wadia, C. et al. 2009. Materials Availability Expands the Opportunity for Large-Scale Photovoltaics Deployment. Environ. Sci. Technol 43: 2072-2077

5 Responses to Why rare and valuable metals are not recycled