Preface. Methane hydrates are far from being commercial, and probably always will be. Scientists and companies have been trying to exploit them since the first energy crisis in 1973 to no avail. Nor are they likely to trigger a runaway greenhouse as I show in “Methane Apocalyse. Not Likely“.
Methane hydrate extraction in the news:
NREL 2021 Japan’s phase 4 methane hydrate research: There is still a long way to go to achieve the project’s goal of introducing marine methane hydrates into the Japanese domestic resource portfolio. The last two phases had operational problems with sand control, flow assurance, and a production rate high enough to be commercially viable. Nonetheless, there will be phase 4 from 2019 to 2022.
Alice Friedemann www.energyskeptic.com Women in ecology author of 2021 Life After Fossil Fuels: A Reality Check on Alternative Energy best price here; 2015 When Trucks Stop Running: Energy and the Future of Transportation”, Barriers to Making Algal Biofuels, & “Crunch! Whole Grain Artisan Chips and Crackers”. Podcasts: Crazy Town, Collapse Chronicles, Derrick Jensen, Practical Prepping, KunstlerCast 253, KunstlerCast278, Peak Prosperity
***
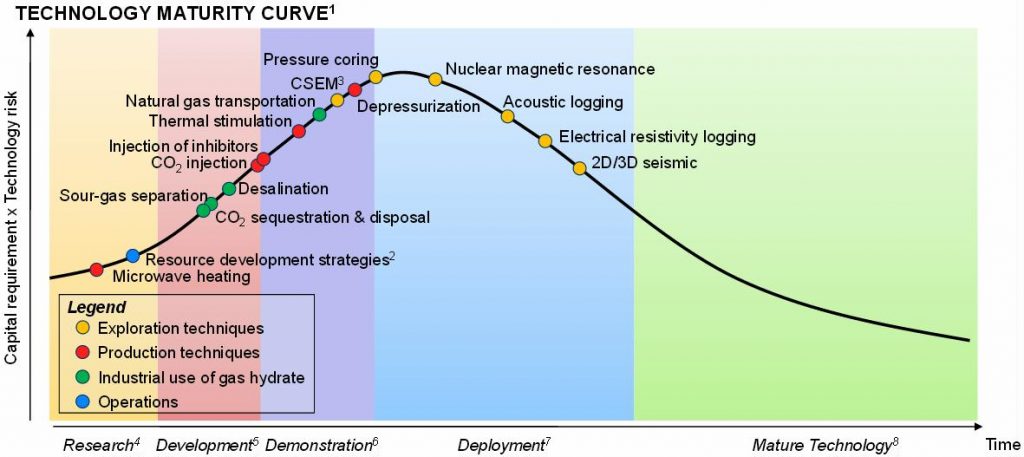
Gas-hydrate technologies remain at an early stage of development, despite the maturity of many of the individual exploration technologies being used. While some technologies may be widely deployed in the conventional oil and gas industry, most are not mature in the context of gas hydrates. For example, while core recovery is common practice in the oil and gas industry, coring technologies had to be adapted to enable gas-hydrate coring, and none of the pressure corers have yet reached a commercial scale; 2 Addressing issues relating to operations, e.g. number and type of wells, and size of drilling vessels; 3 Controlled-Source Electromagnetic Methods; 4 Lab work / theoretical research; 5 Bench-scale; 6 Pilot-scale; 7 Proved commercial-scale process, with optimization work in progress; 8 Commercial-scale, widely deployed, with limited optimization potential. Source: SBC Energy Institute analysis
Methane hydrates are crystalline structures that are mostly water: four methane molecules per 23 water molecules. Methane is trapped within this matrix of ice, so they don’t amass in commercial quantities and the majority are too spread out to harvest for energy.
Their formation depends on low temperatures, high pressures, and water. They’re found 2,000 to 8,000 feet deep in the ocean, often in thin and discontinuous layers, or below 600 to 3,000 foot layers of permafrost in high latitudes.
Big oil companies have known about them since 1970 yet so far haven’t found a way to extract them.
The United States Geological Survey estimates the total energy content of natural gas in methane hydrates is greater than all of the known oil, coal, and gas deposits in the world.
But that’s a wild ass guess since we can’t measure this resource, for reasons such as coring equipment that can’t handle the expansion of the gas hydrate as it’s brought to the surface. And if you do work around this problem, there’s tremendous variability within the same area (Riedel). Since less than 1% of is potentially extractable, there’s no point in throwing around large numbers and getting the energy illiterate excited.
According to petroleum engineer Jean Laherrère, no way do methane hydrates dwarf fossil fuels. “Most hydrates are located in the first 600 meters of recent oceanic sediments at an average water depth of 500 meters or more, which represents just a few million years. Fossil fuel sediments were formed over a billion years and are much thicker — typically over 6,000 meters (Laherrère).
So here it is 2014, with no commercially produced gas hydrate, despite 30 years of research at hundreds of universities, government agencies, and energy companies in the United States, Japan, Brazil, Canada, Germany, India, Norway, South Korea, China, and Russia.
Japan alone has spent about $700 million on methane-hydrate R&D over the past decade (Mann) and gotten $16,000 worth of natural gas out of it (Nelder). I think this reflects the likely EROI of methane hydrates — .0000228 (16000/700,000,000, and yes, I know money and EROI aren’t the same). But EROI doesn’t capture the insanity as understandably as money does. Basically, for every $43,750 you spend, you get $1 back ($700,000,000 / $16,000).
Of course, it’s all theoretical. Maybe you get $500 or $5,000 back. Who knows? There is no commercial production now or in the foreseeable future. And we’ve tried all kinds of thermal techniques to unleash it — hot brine injection, steam injection, cyclic steam, fire flooding, and electromagnetic heating — all of them too inefficient and expensive to scale up to a commercial project (DOE 2009).
Heating them requires just 7% of the energy content released by burning them, the problem is that distributing the heat in the gas hydrate layer because “the normal pore space within the sediments is plugged up by the gas hydrates, so simple injection of a hot fluid into the hydrate layer probably will not work”. Another method would be to convince the water to migrate to a substance more attractive than the methane, an “anti-freeze”. This has been tried with methanol to no effect (Deffeyes).
Even if we found a way to get some of them, they’re so thin and dispersed that the most we could hope for is about 100 Tcfg (trillion cubic feet of gas), about 1% of the present gas URR, despite the fact that the total resources are orders of magnitude higher (Boswell).
1) Gas hydrates are cotton candy crystals mainly found in dispersed, deeply buried impermeable marine shale.
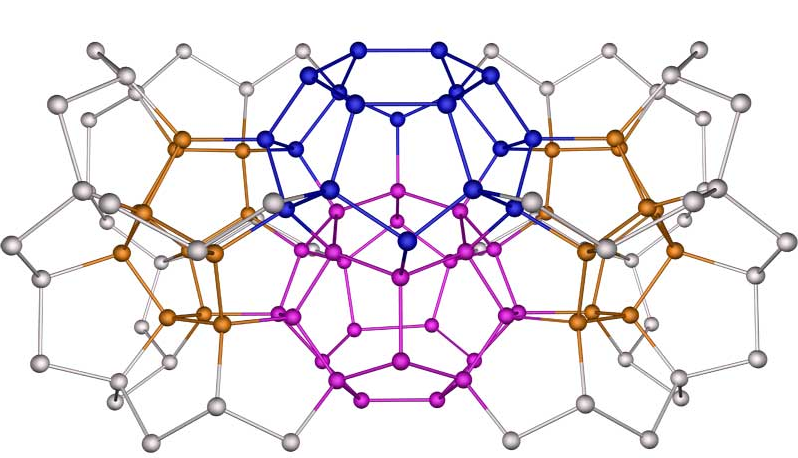
Figure 1. methane hydrate crystals form from dodecahedral clusters of water which create a cage around a single methane molecule. Source: Ken Jordan. 2005. Water Water Everywhere. Projects in Scientific computing.
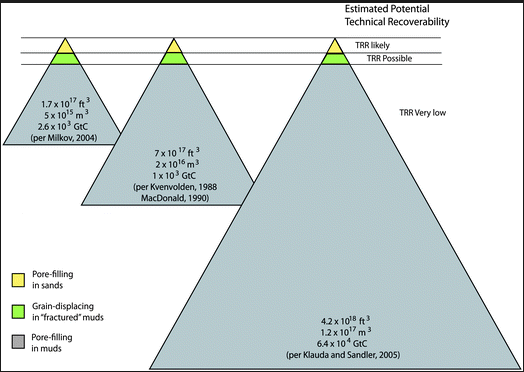
In Figure 2 below, methane hydrates (yellow) in porous sands are the only resource with any chance of being exploited — a very small fraction of the overall methane hydrate resource. Most methane hydrates are locked up in marine shales (gray) where they’ll probably remain forever because:
- The average concentrations are extremely low, about .9 to 1.5% by volume, even in the less than 1% of highly porous sediments where there’s any chance of extracting them
- Marine shales are impermeable, very deep, widely dispersed, with very low concentrations of methane hydrate (Moridis et al., 2008).
- Clathrates are far from oil and gas infrastructure, which you must use to get the methane hydrates stored and delivered
- The infrastructure, technology, and equipment to extract gas hydrates hasn’t been invented yet
- The energy required to get the methane hydrate out has negative Energy Returned on Energy Invested (EROEI). It takes too much energy to heat them in order to release them plus break the bonds between the hydrates’ water molecules.
- Inhibitor injection requires significant quantities of fairly expensive chemicals
Source: Boswell, Ray, et al. 14 Sep 2010. Current perspectives on gas hydrate resources. Energy Environ. Sci., 2011,4, 1206-1215
2) Methane Hydrates are Explosive Cotton Candy
Because as temperature rises or pressure goes down when you bring these ice cubes to the surface, the gas hydrates expand to 164 times their original size. Though most are the size of sugar grains mixed in with other sediments.

Methane hydrates bubbling up to the surface
3) How do you store and get these giant gas bubbles to market?
If you could keep the gas hydrates small, crystalline, and pacified, there would still be that niggling worry you might offend them into their 164-fold fury. So it’s best to let that happen — but now where are you going to store all this gas and how will you deliver it?
You’d have to use oil and gas infrastructure in the Arctic and other questionable places where ownership isn’t settled and potentially create geopolitical tensions.
And imagine how Exxon will feel about that! Their oil rigs are already dodging icebergs. Oil companies avoid drilling through methane hydrates because they can fracture and disrupt bottom sediments, wrecking the wellbore, pipelines, rig supports, and potentially take out a billion dollar offshore platform as well as other oil and gas production equipment and undersea communication cables.
4) The Mining of Gas Hydrates can cause Landslides…
Eastman states that normally, the pressure of hundreds of meters of water above keeps the frozen methane stable. But heat flowing from oil drilling and pipelines has the potential to slowly destabilize it, with possibly disastrous results: melting hydrate might trigger underwater landslides as it decomposes and the substrate becomes lubricated…
5) Which can Trigger Tsunamis
Landslides can create tsunamis that migh result in fatalities, long term health effects, and destruction of property and infrastructure.
6) Methane Hydrates are a greenhouse gas 23 times more potent than carbon dioxide
Climate scientists like James E. Hansen worry that methane hydrates in permafrost may be released due to global warming, unleashing powerful feedback loops that could cause uncontrollable runaway climate change.
Scientists believe that sudden, massive releases of methane hydrates may have led to mass extinction events in the past.
Considering that the amount of methane onshore and offshore could be 3,000 times as much as in the atmosphere, it ought to be studied a bit more before proceeding, don’t you think? (Whiteman 2013, Kvenvolden 1999).
7) Ecological Destruction
They’re dispersed across vast areas at considerable depths, which makes them very ecologically destructive to mine, since you have to sift through millions of cubic yards of silt to get a few chunks of hydrate.
8) Toxic Waste
The current state of technology uses existing oil drilling techniques, which generate wastes including produced formation water (PFW), drilling fluid chemicals, oil and water-based drilling muds and cuttings, crude oil from extraction processes and fuel/diesel from ships and equipment (Holdway 2002).
9) EROI
There are only two studies on EROI, both by Callarotti, and he looks only at the heat energy used to free the clathrates up, and it’s published in a journal called Sustainability that would better be named Gullibility when it comes to the topic of energy which is not their specialty. He comes up with an EROI of 4/3 to 5/3 using just that one parameter. Callarotti knows this is a dishonest figure because he says “If one were to consider the energy required for the construction of the heaters, the pipes, and the pipe and the installation process, the total EROI would be even less.”
Is he kidding? What about the energy used to mine and crush the ore to get the metals to build the pipelines, drilling, dredging and sifting through the sediment equipment, methane hydrate processing plant, the vessel and the diesel burned to get to the remote (arctic) location, and so on.
10) Technical challenges (House 2009)
Gas hydrate wells will be more complex than most conventional and unconventional gas wells due a number of technical challenges, including:
- Maintaining commercial gas flows with high water production rates
- Operating with low temperatures and low pressures in the well-bore
- Controlling formation sand production into the well-bore
- Ensuring well structural integrity with reservoir subsidence
Technologies exist to address all of these issues, but will add to development costs. Gas hydrate development also has one distinct challenge compared to other unconventional resources, and that is the high cost of transportation to market.
Most gas fields require some compression to maximize reserve recovery, but this typically occurs later in the life of the field after production starts to fall below the plateau rate. For a gas hydrate development, the required pressure to cause dissociation will require the use of inlet compression throughout the life of the field including the plateau production time. This will require a larger capital investment for compression at the front end of the project, and will also result in higher operating costs over the life of the project.
Water production is not uncommon in gas wells, however water rates are typically less than say 10 bbls/MMscf (barrels of water per million standard cubic feet of gas) for water of condensation and/or free water production. Wells that produce excessive amounts of water are typically worked-over to eliminate water production or shut-in as non-economic. The water production from a gas hydrate reservoir could be highly variable, however water:gas ratios in excess of 1,000 bbls/MMscf are possible. This water must be removed from the reservoir and wellbore to continue the dissociation process. On this basis, a gas hydrate development will require artificial lift such as electric submersible pumps or gas lift, which will also increase capital and operating costs over the life of the field. But it is important to highlight that the water in gas hydrate contains no salts or impurities, it is fresh water and may be a valuable coproduced product of a gas hydrate development.
The combination of low operating pressures and high water rates will require larger tubing and flowlines for a gas hydrate development, in order to minimize friction losses and maximize production. Additional water handling facilities and water disposal will also be required. Larger inhibitor volume (such as glycol) will be required to prevent freezing and hydrate formation in tubing and flow-lines. Other items such as sand control, reservoir subsidence, down-hole chemical injection, possible requirements for near well-bore thermal stimulation, etc., will also require additional capital and operating costs for gas hydrate developments compared to conventional gas developments.
Onshore gas hydrates in North America are located on the North Slope of Alaska and on the Mackenzie Delta in Canada. These resources, along with significant volumes of already discovered conventional gas, are stranded without a pipeline to market. In order to compete for pipeline capacity, the economics of onshore gas hydrate developments must be attractive at prevailing gas prices.
By all estimates, the majority of gas hydrates considered for production are located in sandstone reservoirs in deepwater environments. Deepwater drilling technology and experience continues to evolve, and the worldwide deepwater fleet continues to expand. However the deepwater environment is still a very high cost and very high risk area of operation. Offshore gas hydrate developments must have strong economic drivers in order to compete with other deepwater exploration and development opportunities. Adding on the risk of gas hydrates is yet another level of risk to add onto the existing high-risk drilling in deep water.
Significant scientific and exploration work must be completed before gas hydrates can be considered as a viable source of natural gas. Critical among these tasks remains the validation reservoir and well performance through extended field testing that demonstrates the ability to produce gas hydrates at commercial rates with current technology.
So far the small-scale experiments have not been able to bring gas hydrates as far as the surface of the ocean.
On the basis of the studies done to date, gas hydrate developments will have capital and operating costs significantly higher than other unconventional or conventional developments due to well productivity, low operating pressures and temperatures, and high water production rates. Surface facilities for gas hydrate developments will also be higher due to the requirements for larger surface flowlines and inlet facilities (required because of low pressures and water production rates) and the requirement for inlet compression into the processing plant.
The reason methane hydrate production rates peak in later years, while conventional natural gas wells peak immediately is because unconventional hydrocarbons are so called because they are found in
formations other than the typical sandstone or carbonate reservoirs i.e. extremely low permeability or tight,reservoirs, shale, or coal beds the hydrocarbons are in their normal fluid condition and can typically flow without undergoing a fundamental change (except of course for bitumen). The types of reservoirs targeted for gas hydrate testing (and eventual development) are relatively high permeability conventional sandstone reservoirs however the methane gas is locked in a solid gas hydrate crystal so actually the gas is unconventional, not the reservoir. Based on simulation studies, the maximum gas production rate therefore occurs not on days one as with conventional gas reservoirs, but some time into the future, typically years.
All gas reservoirs, conventional or unconventional, are capable of their maximum rate on day one of operation. This is because the reservoir pressure is at its maximum (average reservoir pressure declines with production for most reservoirs), the gas that initially flows into the well is in the near wellbore area, and of course the gas is continuous throughout the reservoir. As gas production continues the gas that flows into the wellbore flows through the reservoir rock from greater and greater distances away. Flowing gas through the reservoir rock results in additional pressure loss, and the production rate begins to decline. Some gas wells in high permeability conventional reservoirs can flow at a more or less constant rate or steady state condition for some time, but eventually the production rate will decline. Unconventional gas reservoir production rates typically decline quite rapidly, and may never actually reach any sort of steady state production, although the rate of decline will drop and the wells may produce for many years. At the start of production for a gas hydrate reservoir, there is no free gas in the reservoir it is all locked up in the hydrate crystals in the pores space of the reservoir rock. The hydrate must first be dissociated, and then the water and free gas can flow to the well. Because water and gas is flowing simultaneously (termed multi-phase flow), the pressure loss through the reservoir will be higher than if just gas only was flowing. Gas and water saturations through the dissociated region will change with time, and gravity will affect the gas and water phases, therefore the flow mechanism will be quite complex.
Conclusion
You don’t have to be a scientist to see how difficult the problem is:
- Somehow you’ve got to capture the energy in thousands of square miles of exploding grains of sugar that erupt into a gas 164 times their size.
- There are huge deposits of natural gas that are easier to get at and far more valuable that aren’t being exploited because they’re stranded (not near pipeline infrastructure), so who’s going to invest in a resource of much lower quality at the bottom of the pyramid with such dismal prospects?
- We can’t even drill for oil in most of the Arctic (Patzek) which is where a lot of the methane hydrates are, and that infrastructure has to be there to even think of trying to get at the methane hydrates.
- Most of the hydrates are in a thin film on the deep ocean floor. Are you going to build a thousand square mile blanket to trap the bubbles like a school of fish? Or use expensive fracking & coalbed methane techniques?
- Permafrost gas hydrate is so shallow there’s not enough pressure to get it to flow fast enough to be worth mining
Gas hydrates are stranded in distant regions and deep oceans. It would be far cheaper to go after large natural gas reservoirs than attempt to go after mostly small deposits of methane hydrates we don’t even know how to extract yet.
Despite all the happy talk that says we can meet these challenges by 2025 if only there were more funding, we’re out of time.
It’s highly unlikely that Methane Hydrates will ever fuel the diesel engines that do the actual work of civilization, all of them screaming “Feed Me!” as oil declines in the future.
References
Arango, S. O. May 7, 2013. Canada drops out of race to tap methane hydrates Funding ended for research into how to exploit world’s largest fossil energy resource. CBC News
Benton, Michael J. 2003. When Life Nearly Died: The Greatest Mass Extinction of All Time. Thames & Hudson.
BBC. 5 December 2002. The Day The Earth Nearly Died. Permian-Triassic Extinction Event
Boswell, R. 2009. Is gas hydrate energy within reach? Science.
Callarotti, R. C. 2011. Energy Return on Energy Invested (EROI) for the Electrical Heating of Methane Hydrate Reservoirs. sustainability 2011, 3.
Collett T. S. April 19-23, 2002. “Detailed analysis of gas hydrate induced drilling and production hazards,” Proceedings of the Fourth International Conference on Gas Hydrates, Yokohama, Japan.
Carrington, Damian. 23 Nov 1999. Fossil fuel revolution begins.
Deffeyes, K.S. 2005. Beyond Oil. The View from Hubbert’s Peak. Hill and Wang.
DOE 2009. U.S. Department of Energy. 2009. International Energy Outlook 2009
Eastman, Q. 2004. Energy Saviour? Or Impending Disaster? Science Notes.
Holdway, D. A. 2002. The acute and chronic effects of wastes associated with offshore oil and gas production on temperate and tropical marine ecological processes. Marine Pollution Bulletin, Vol 44: 185-203.
House. 2009. UNCONVENTIONAL FUELS PART II: THE PROMISE OF METHANE HYDRATES. U.S. House of Representatives.
Jayasinghe, A.G. 2007. Gas hydrate dissociation under undrained unloading conditions. P. 61 in Submarine Mass Movements and Their Consequences. Vol. IGCP-511. UNESCO.
Kaneshiro-Pineiro, M. et al. Dec 4, 2009. Report on the Science, Issues, Policy, and Law of Gas Hydrates as an Alternative Energy Source. East Carolina University. Coastal Resources Management Program.
Kvenvolden, K.A. 1999. Potential effects of gas hydrate on human welfare. Proceedings in the National Academy of Science. USA. 96: 3420 – 3426.
Laherrère, Jean. July 17, 2009. Update on US Gulf of Mexico: Methane Hydrates. theoildrum europe.
Mann, C. C. May 2013. What If We Never Run Out of Oil? New technology and a little-known energy source suggest that fossil fuels may not be finite. This would be a miracle—and a nightmare. The Atlantic.
Moridis, George. 2006. “Geomechanical implications of thermal stresses on hydrate-bearing sediments,” Fire in the Ice, Methane Hydrate R&D Program Newsletter.
Moridis, G.J., et al. 2008. Toward production from gas hydrates: Current status, assessment of resources, and simulation-based evaluation of technology and potential. Paper SPE 114163.Presented at the SPE Unconventional Reservoirs Conference, Keystone, Colo., February 10–12, 2008.
Nelder, C. 2013. Are Methane Hydrates Really Going to Change Geopolitics? The Atlantic.
Office of Naval Research. 5 Nov 2002. Fiery Ice From The Sea: A New World Energy Source?
NAS 2009. America’s Energy Future: Technology and Transformation. 2009. National Academy of Sciences, National Research Council, National Academy of Engineering.
Patzek, Tad. 29 Dec 2012. Oil in the Arctic. LifeItself blog.
Riedel M and the Expedition 311 Scientists. 2006. Proceedings of the IODP, 311: Washington, DC (Integrated Ocean Drilling Program Management International, Inc).
Whiteman, G. et al. 25 July 2013. Vast costs of Arctic change. Nature, 499, 401-3.