Preface. My book, When Trucks Stop Running, makes the case that civilization ends when trucks stop running. The replacement for diesel fuel that everyone expects, especially because Elon Musk has told them it’s on the way, are battery electric trucks. But the Semi is years late, and not going to solve the problem, it’s only meant to run on smooth roads. What’s really needed are off-road electric tractors and harvesters, logging, mining, construction, and other essential trucks.
There are many other barriers besides a limited number of elements to choose from in the periodic table to building a battery for transportation (or utility scale energy storage). Vehicles use many finite platinum group elements, precious elements, and rare earth elements. Plus there are dozens of challenges to improving batteries that must be overcome but can’t because of the laws of physics and thermodynamics. Nor are trucks going to be running on hydrogen: The dumbest & most impossible renewable.
Alice Friedemann www.energyskeptic.com Author of Life After Fossil Fuels: A Reality Check on Alternative Energy; When Trucks Stop Running: Energy and the Future of Transportation”, Barriers to Making Algal Biofuels, & “Crunch! Whole Grain Artisan Chips and Crackers”. Women in ecology Podcasts: WGBH, Jore, Planet: Critical, Crazy Town, Collapse Chronicles, Derrick Jensen, Practical Prepping, Kunstler 253 &278, Peak Prosperity, Index of best energyskeptic posts
***
There hasn’t been much progress in batteries the past 200 years. Electric cars still cost about twice as much as gasoline cars. But who cares about cars? Civilization depends on heavy-duty trucks, rail, and ships that are the basis of all supply chains, mining, agriculture, logging, construction industries, and infrastructure.
Batteries are simply not as energy dense as oil and never will be. Pound for pound, oil is 500 times more energy dense than a lead-acid battery and about 120 times more than a lithium-ion battery. That makes them too heavy to move a heavy-duty truck or other large vehicle.
The periodic table limits any possibility of a major breakthrough, because there are only 118 possible elements, and most of them can be ruled out:
- 39 are radioactive
- 23 are far too scarce or expensive to scale up commercially such as rare earth and platinum group metals
- 6 inert noble gases
- 4+ toxic metals such as cadmium, cobalt,mercury, arsenic
- too heavy
- too scarce
- too valuable (i.e. gold, platinum group metals)
- too hard to recycle
- too little reduction or oxidation potential
One of the main problems with batteries is that they’re too heavy, especially for the heavy-duty vehicles and equipment that civilization depends on.
We’ve already gained as much energy density as possible by switching to lighter and lighter elements — from lead to zinc to nickel to lithium. Consider that when you hear about yet another battery improvement.
There’s nowhere to go from here, lithium is the lightest element we can make batteries out of, with only hydrogen and helium being lighter. Lithium is much lighter than lead at 82, zinc at 30, and nickel at 28.
At the very best, scientists estimate that we could get double or triple lithium-ion performance due to the laws of physics.
Nor is there enough lithium in the world to switch from gas and diesel vehicles to electric vehicles running on lithium batteries (Vikström, H. et al. 2013). And especially not if batteries are built to store energy for when the wind and sun aren’t out. On average at least six weeks of energy storage would be needed for a 100% renewable grid. To store just one day of U.S. electricity generation, Li-ion batteries would cost $11.9 trillion dollars, take up 345 square miles, and weigh 74 million tons (DOE/EPRI 2013). There are other battery types, but commercial development is focused on lithium almost entirely.
The very heavy, 4,647 pound Tesla Model S gets most of its mileage from aerodynamics, reduced rolling resistance, light-weight materials, and so on. The Tesla S goes further than other all-electric cars because it has more batteries. Tesla S battery packs weigh 1,323 pounds (plus 350 lbs for the electric motor and inverter) versus 660 lbs for the Nissan Leaf (also pretty heavy at 3,340 lbs).
So let’s start over and design a high-energy battery from scratch. The first step is to look at the periodic table to choose the best elements.
Here is Aidan Stranger’s point of view (taken from a comment below): “Alkali metals (and indeed most other metals) have a greater reducing potential the further down the periodic table you go. So if reactivity were the deciding factor, caesium would be the best choice (francium’s not an option because it’s radioactive, extremely rare and very short lived). If cost is the deciding factor, sodium’s a better choice. But lithium is usually preferable for batteries because it’s lightweight; the higher specific energy more than makes up for the lower energy density. But there are more factors to consider. Alkali metals only have one electron each. Something with more outer shell electrons could be more effective. Vanadium (with five) is often used in wet cells. As for fluorine, forget it! Fluorine gas is far too dangerous to have in batteries, and oxygen fluorides are also dangerous and difficult to work with. I’m amazed that anyone’s even contemplated it. Unlike fluorine, cadmium can be contained fairly easily, so has been used for batteries despite its toxicity.”
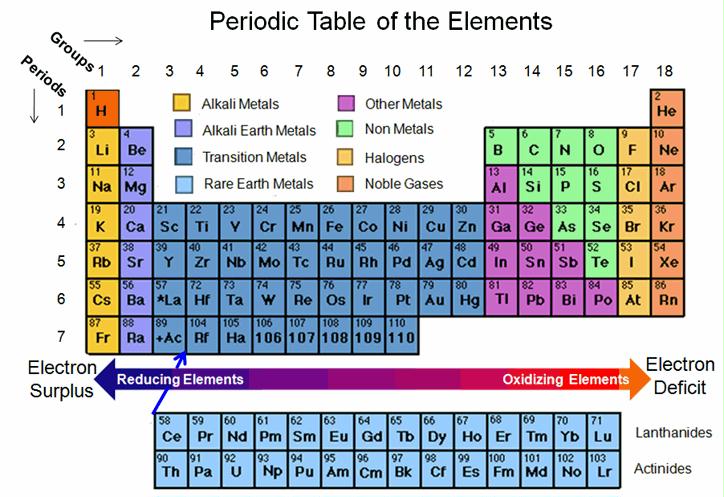
Another possibility is looking at what elements could produce the highest voltage from the most reducing and most oxidizing elements. The highest potential is nearly 6 volts with a lithium anode (the strongest reducing element) and a fluorine cathode (strongest oxidizing element) of -3.04 & 2.87 respectively). Battery researchers know this and have been trying to develop such a battery since the 1960s. Scrosati et al have an excellent history of Li-F battery research and where we stand on different battery types today if you want to know the technical details.
The material electrons swim through between the anode and cathode matters as well. Cells with aqueous (containing water) electrolytes are limited to less than 2 volts because the oxygen and hydrogen dissociate above this voltage. This is a shame, because water is very inexpensive. Lithium batteries don’t use water but this prevents electrons from flowing as well (high internal impedance) so 2.7 to 3.7 volts are achieved, rather than the maximum 6 volt potential between lithium and fluorine
The laws of physics means that there is no possibility of making a battery that rivals the energy density of oil, ever. So the question is, if a 6 volt lithium-fluorine battery could be built — and it probably can’t — but if it could, would it be energetically cheap and powerful enough to move heavy-duty class 7 & 8 all-electric tractors, harvesters, road construction, and mining trucks?
If so, then is there enough lithium on the planet, including recycling, to make enough batteries for all electric cars, trucks, and utility-scale energy storage and lithium-battery mining trucks to haul ore to refining plants, and so on?
The main elements that lithium are dating are shown below. The rare earth elements aren’t in the battery, but are being used in the electric motor and generator, so even if lithium is recycled, the limits to EV may come from rare earth metals used other components of electric vehicles, which can NOT always be recycled: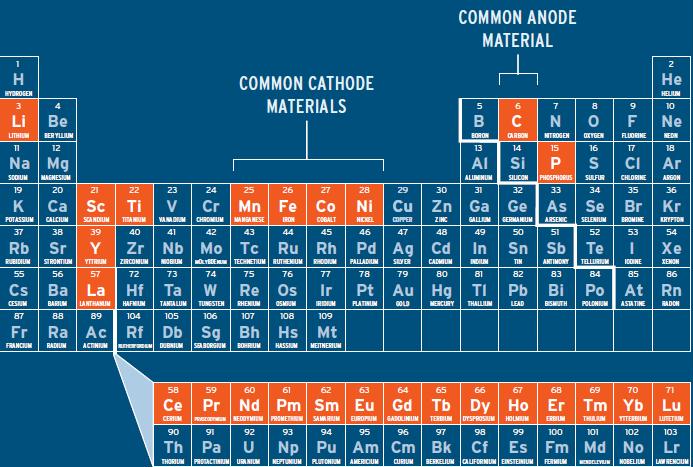
Related Posts: The electric grid will eventually fail without utility scale energy storage of at least a month of electricity to compensate for seasonal deficits (When Trucks Stop Running Chapter 17 The Electric Blues). Natural gas is the main energy storage now (and coal), and essential for balancing the sudden life and death of wind and solar power. But natural gas and coal are finite. Yes, hydropower can also balance wind and solar, but mostly in the 10 lucky states that have 80% of it for just part of the year, and the few places that can afford multi-million-dollar batteries (though only for an hour or so). The electric grid could crash from a weapon or solar flare electromagnetic pulse and be down for a year or more. Electric trucks are impossible. Without trucks, civilization fails. And it’s checkmate as well, because manufacturing uses over half of all fossil fuels, and depends on the high heat only fossils can provide to make cement, steel and other metals, glass, brick, ceramics, microchips and so on. Manufacturing can’t be run on electricity, hydrogen, or anything else, as explained in Chapter 9 of Life After Fossil Fuels. No transportation? No Manufacturing? Then no electricity generating contraptions like solar panels or wind turbines can be built. Checkmate.
References
DOE/EPRI. 2013. Electricity storage handbook in collaboration with NRECA. USA: Sandia National Laboratories and Electric Power Research Institute.
Scrosati, B., et al. 2013. Lithium batteries. Advanced technologies and applications. Wiley.
Vikström, H. et al. 2013. Lithium availability and future production outlooks. Applied Energy, 110(10): 252-266.
For a more in-depth look at battery chemistry see: Battery and Energy Technologies. Cell Chemistries – How batteries work. Electropaedia. mpoweruk.com

7 Responses to The periodic table limits battery development