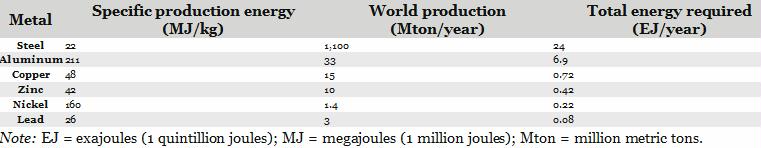Preface. This is a small section of Ugo Bardi’s excellent book “Extracted”.
His most important observation is that:
“The limits to mineral extraction are not limits of quantity; they are limits of energy. Extracting minerals takes energy, and the more dispersed the minerals are, the more energy is needed. Today, humankind doesn’t produce sufficient amounts of energy to mine sources other than conventional ores, and probably never will.
But like all minerals, long before they “run out”, if oil peaks, then game over, fossil fuel resources are necessary for the extraction of almost everything else, and the easy high-grade ores have been mined, leaving crummy ore and expensive declining fossils left to extract it.”
Bardi also explains why we can’t extract copper, uranium, or any other metal from the ocean.
Alice Friedemann www.energyskeptic.com author of “When Trucks Stop Running: Energy and the Future of Transportation”, 2015, Springer, Barriers to Making Algal Biofuels, and “Crunch! Whole Grain Artisan Chips and Crackers”. Podcasts: Collapse Chronicles, Derrick Jensen, Practical Prepping, KunstlerCast 253, KunstlerCast278, Peak Prosperity , XX2 report
***
Bardi, Ugo. 2014. Extracted: How the Quest for Mineral Wealth Is Plundering the Planet. Chelsea Green Publishing.
The average crustal abundance of elements such as copper, zinc, lead, and others is below 0.01 percent in weight (100 parts per million). Some very rare elements, such as gold, platinum, and rhodium, exist in the crust as a few parts per billion or even less. However, most rare elements form specific chemical compounds that can be found at relatively high concentrations, called deposits, in certain regions. As we know, some of those deposits that are concentrated enough that we can actually extract minerals from them are called ores.
Mining ores is a multistage process. The first is the extraction phase, in which materials are extracted from the ground. Then follows the beneficiation stage, when the useful minerals are separated from the waste (also known as gangue). Further processing stages normally follow; for instance, the production of metals requires a smelting stage and a refining one. All these stages require energy. Table 1 lists the specific energy needed for the production of some common metals, together with the total energy requirement for the present world production.
Table 1. Energy required for production of some common metals.
From this table, we can see that the world’s production of steel alone requires 24 exajoules, equivalent to about 5% of the world’s total primary energy production (about 450 exajoules). Also note that, today, we extract copper from ores that contain it in concentrations of 0.5 to 1 percent. The total energy involved is 50 megajoules per kilogram. Using this value, we find that we need about 0.7 exajoules for the world’s copper production. This is about 0.2 percent of the world’s total energy production.
Taken together, the data of the table indicate that the total energy used by the mining and metal-producing industry might be close to 10% of the total world energy production—an estimate consistent with other projections.
As we run out of high-grade ores, we have to move to lower-grade ores.
In general, the lower the ore grade, the more energy is needed for extraction. For example, if an ore has a mineral concentration that is 10 times lower than another, it will take 10 times more energy to extract that mineral from the ore. This is an approximation, especially when applied to the whole production process that includes smelting and refining. But we can take it as a reasonable “first order” approximation. We saw that we are already committing about 10% of the world’s primary energy to the production of minerals. This amount can only increase as we access lower-grade resources, even if we are aiming at just maintaining the present production levels. Therefore, if we want to maintain the current fraction of energy allocated to the mining industry, we must increase the world’s total energy production in proportion. That has been possible, so far, by increasing the production of fossil fuels, but it is becoming more and more difficult. The problem of dwindling ore grades occurs also with fossil fuels; energy is becoming more and more energy-expensive to produce. Nevertheless, the extra energy needed to access low-grade ores must come from somewhere, and at present it is being drawn from other sectors of the economy. That can’t be painless, and the pain appears in the present trend of rising prices for all mineral commodities.
Copper is present at very small concentrations, about 25 parts per million, in the upper crust. To produce 1 kilogram of copper from the undifferentiated crust, we would need to process 40 tons of rock.
The average American home consumes about 9,000 kilowatt-hours per year of electric energy, or 32,400 MJ.
Antarctica is the only major continent still unexplored for mineral resources, and there are most likely ores there. But at present finding or extracting anything that exists under kilometers of ice is an unthinkable endeavor.
Underwater mining requires complex and expensive technologies. The high costs involved may be justified only in the case of very valuable minerals, such as offshore diamond mines. That is done, for instance, off the coast of Namibia. 21 In some cases it is possible to mine undersea deposits as an extension of conventional mines, as is done in Japan for some coal mines. 22 It is often possible to extract oil and gas from the continental shelf because the process of offshore drilling can be completely automated and is not much different than it is on land—except for the need for a floating platform for hosting the drilling equipment. Of course, this kind of drilling carries risks that are not seen on land, as when the Deepwater Horizon drilling platform operating in the Gulf of Mexico exploded in 2010, releasing huge amounts of oil into the ocean ecosystem.
In general, sea floor deposits are too dispersed and at concentrations too low to be commercially interesting, even without considering the energy and monetary cost of mining at such great depths.
The problems with extracting minerals from seawater are twofold: the limited amounts available and the energy requirement. Calculations of these parameters are not encouraging. The oceans are vast, but rare metals are dissolved in them in extremely tiny amounts. In the case of copper, for instance, there is about 1 billion tons of it in the form of copper ions dissolved in the whole mass of seawater on the Earth. That may seem to be a large amount, but consider that we now produce about 15 million tons of copper every year. Even if we were able to filter the whole mass of all the oceans—an unlikely prospect (also very bad from the viewpoint of fish, whales, and all other sea creatures)—we would run out of oceanic copper in little more than 60 years.
Extracting ions dissolved in water doesn’t require the energy-expensive process of rock breaking, lifting, and crushing of conventional mining. However, the concentrations of rare metal ions in seawater are enormously smaller than they are in mineral ores. So extracting a specific ion from seawater requires filtering enormously large amounts of water. That is not just a practical problem; it takes energy to pump water through a filtering membrane or, alternatively, for all the operations needed to transport the membrane to sea, leaving sea currents to move water in and out, and then to recover it.
Uranium extraction from seawater is still discussed as a future possibility. However, it is possible to calculate that the energy needed to extract and process uranium from seawater would be about the same as the energy that could be obtained by the same uranium using the current nuclear technology. That, of course, would make extraction from seawater useless.
Lithium recycling is almost non-existent – less than 1% globally partly because it’s cheaper to mining it than to recycle it.
Mining the solar system. Even if it turns out that asteroids and other planets have minerals we want, “the energy cost needed to reach them, mine ores, and then bring back the minded materials to earth is truly out of this world.
Nickel and zinc have limited exploitable deposits, so the problem of depletion cannot be ignored.
More than 12,000,000 tons of zinc are mined a year, the 6th most used metal. The average ore grade decreased from 7 to 5.5% between just 2000 and 2012. The “official” current reserves represent 20 years of production.
Over 1,800,000 tons of nickel are mined a year, putting it in 10th place. Nickel reserves-to-production ration yields estimate 45 years of supply at current production rates.
Dissipation of minerals makes them unavailable for recycling. For example, zinc oxide in toothpaste won’t be recycled at a water treatment plant or reclaimed when used as a white pigment or additive in plastics or glass. When used in car tires, infinitesimal amounts are left on the pavement, and the rest is lost in landfills or ashes when incinerated.
After collapse, the remaining population will have plenty of metals though. And once we’ve switched from cars to bicycles and horses, we’ll have little need for most of the high-tech ways we use many metals today.
Depletion is unavoidable
Perhaps this is why we have low oil prices now]: Jevons and Hotelling emphasized that over a certain limit, rising prices cause a reduction in demand, and that eventually stops rising production. Industry won’t extract resources so expensive they’re impossible to sell. Consequently, there’s a limit to the low-grade resources the industry can exploit. Economists assume that technology will always come to the rescue, lower costs of extraction and restoring both demand and industry profits. But this is a leap of faith: technology has monetary and energy costs so there are limits to what it can do.
And one thing is for sure: no technology can extract minerals that are not there.
The metaphor of Achilles’ heel is often used when a large and apparently solid structure fails because of a critical defect. Petroleum could be the Achilles’ heel of modern society. It could be argued that phosphorus is even more essential than oil.
Optimism about the depletion problem comes from a basic mistake – that of considering the amounts of minerals available and not the energy cost of recovering them.